
Researchers at the SLAC-Stanford Battery Center recently discovered that charging lithium-ion batteries at high currents during their initial formation significantly extends their lifespan by 50%. The study, led by Professor Will Chueh and published in the journal Joule, revolutionizes battery manufacturing processes and efficiency.
“This is an excellent example of how SLAC is doing manufacturing science to make critical technologies for the energy transition more affordable,” says Professor Chueh. He emphasizes the practical implications of this discovery for the energy sector, noting that it addresses a significant challenge faced by the industry.
Innovating Conventional Methods
The research, in collaboration with the Toyota Research Institute (TRI), Massachusetts Institute of Technology (MIT), and the University of Washington, focuses on the formation charge, a crucial initial step in lithium-ion battery production.
Traditionally, this process involves a slow, low-current charge over 10 hours to minimize lithium loss, which forms a protective layer called the solid electrolyte interphase (SEI) on the negative electrode. The SEI layer is vital for protecting the electrode from side reactions that degrade the battery over time.
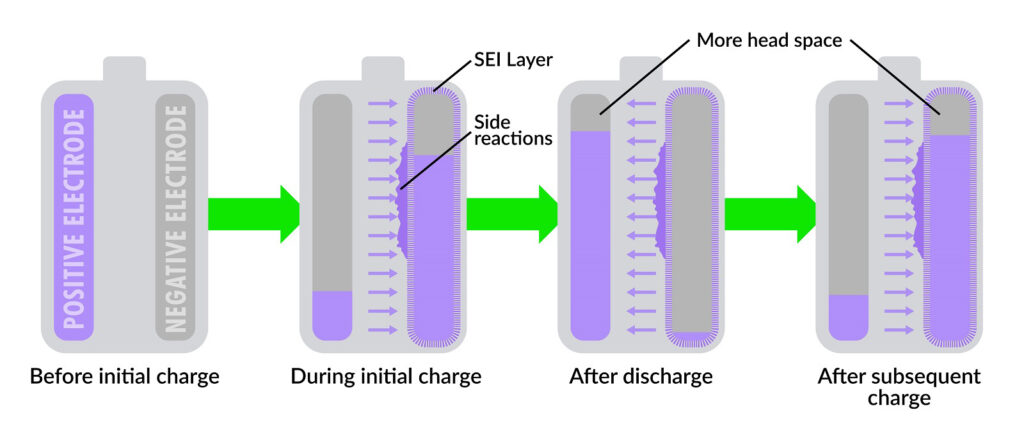
Image Source: SLAC
However, the SLAC-Stanford team challenges this convention by applying a high current during the first charge and reducing the initial charging time from 10 hours to just 20 minutes. This method leads to a higher initial loss of lithium—about 30%, compared to the 9% loss with the traditional method. Despite this, the high-current approach results in a better SEI layer, ultimately extending the battery’s lifespan by an average of 50%.
Xiao Cui, the lead researcher in Chueh’s lab, explained that, oddly enough, a method to reduce lithium depletion involves intentionally allowing a significant portion of the initial lithium content to be expended during the battery’s initial charging process.
This counterintuitive strategy, Cui notes, creates additional headspace in both electrodes, enhancing battery performance and longevity. It is similar to scooping water out of a full bucket before transporting it.
Machine learning, a well-established field in artificial intelligence, also plays a pivotal role in this study, allowing the researchers to pinpoint significant features in the battery electrodes that affect the increased lifespan and performance.
Notably, two factors—the temperature and the charging current of the battery—were identified as the most significant.
“Brute force optimization by trial-and-error is routine in manufacturing—how should we perform the first charge, and what is the winning combination of factors?” Chueh said. “Here, we didn’t just want to identify the best recipe for making a good battery; we wanted to understand how and why it works. This understanding is crucial for finding the best balance between battery performance and manufacturing efficiency.”
Implications for Manufacturing and Electric Vehicle Industry
Steven Torrisi, senior researcher at the TRI, highlights the broader implications of this research. “Battery manufacturing is extremely capital, energy, and time intensive. It takes a long time to spin up manufacturing of a new battery, and it’s really difficult to optimize the manufacturing process because there are so many factors involved,” he says. Torrisi believes that the findings from this study could be transferred to new processes, facilities, equipment, and battery chemistries in the future.
This research is particularly significant for the electric vehicle (EV) industry. As the demand for EVs continues to surge, manufacturers are under pressure to produce batteries that are not only efficient but also durable.




















